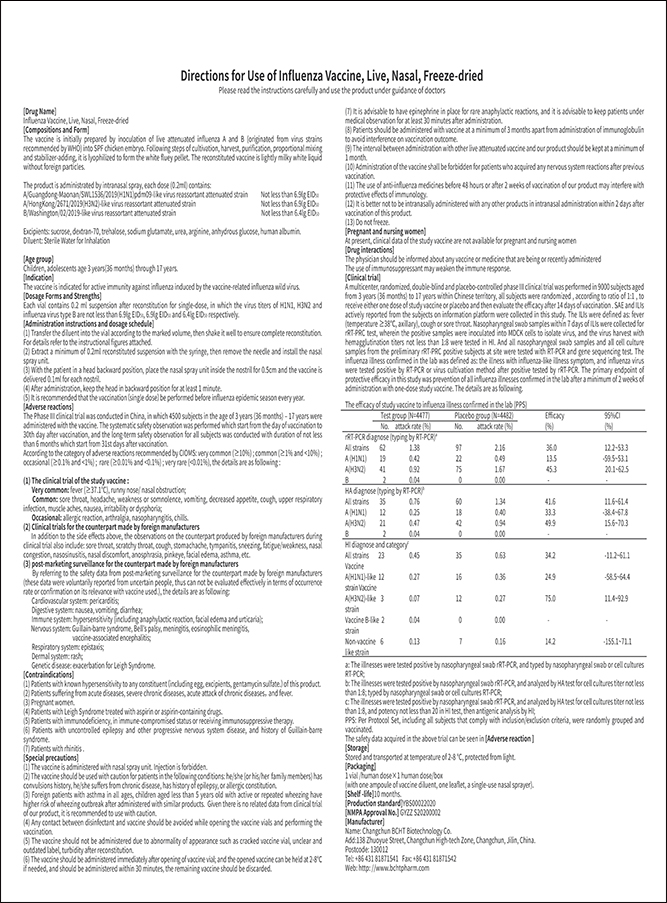
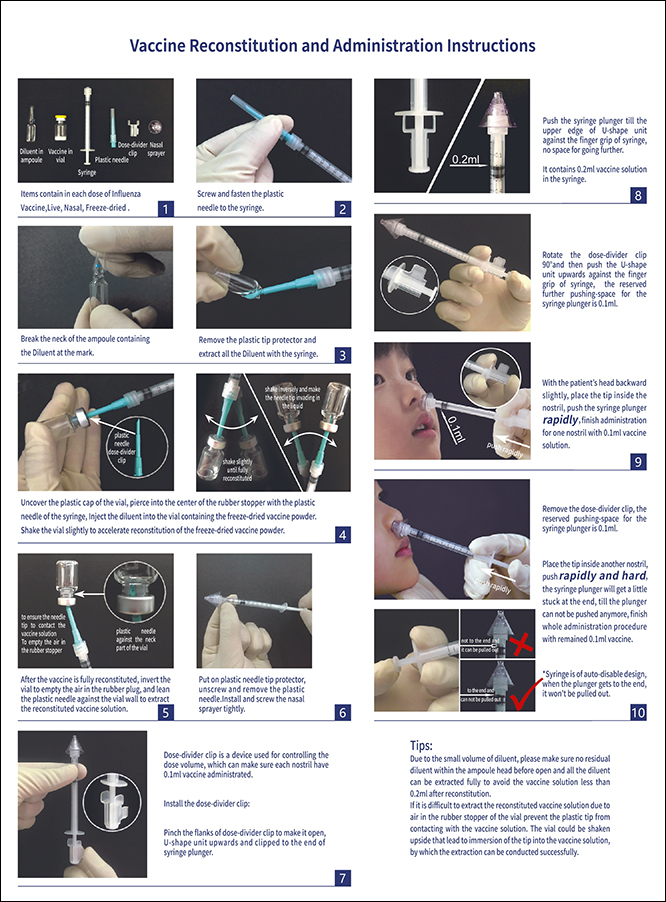
Product Name: Influenza Vaccine, Live, Nasal, Freeze-dried
Approval No.: S20200002
Brief Introduction:
●Exclusively authorized by the WHO in China
Influenza Vaccine, Live, Nasal, Freeze-dried is a cooperative project with the WHO, which has been included in Global Action Plan for Influenza Vaccines (GAP). Hundreds of millions of doses have been used in the world, and Changchun BCHT Biotechnology Co. has the exclusive right of production and operation in China.
●Mucosal immunity + Cellular immunity + Humoral immunity
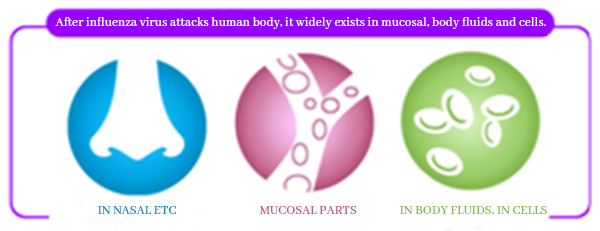
After influenza virus attacks human body, it widely exists in nasal cavity, respiratory tract and other mucosal parts, as well as in body fluids and cells. Vaccination of Influenza Vaccine, Live, Nasal, Freeze-dried can quickly stimulate the triple immune response of human body, and carry out defense against viruses in different parts:
▼Intranasal administration can produce mucosal immunity (IgA antibody), which forms the first immune defense line in the nasal cavity.
▼Produce humoral immunity (IgG antibody) to remove influenza virus from body fluids.
▼Produce cellular immunity (T cells) to remove influenza virus from cells
●3+N More extensive protection
The production strains are recommended and supplied by the WHO every year. The vaccine can not only effectively resist the vaccine strain, but also produce cross immunity to other subtypes of influenza virus.
●Produced with SPF embryonated eggs
The chick embryos for vaccine production comes from SPF (specific pathogen free) chicken flocks, so the risk of exogenous pathogenic microorganism pollution is excluded.
●Free of inactivator, split agent and preservative
●High protection and fast antibody production
This is the first influenza vaccine in China that has been evaluated by the protection effect of etiology in clinical stage. The protective effect of phase III clinical trial is consistent with the literature.
First, the immune barrier was formed in the nasal mucosa after LAIV vaccination. It has been reported that the antibody of nasal mucosa can be produced in 3 days, which greatly shortens the time for vaccine to produce protective effect.